
METU HS Ankara 2015 Team
VARROA CULA


Behavior Experiment
In this experiment we test effects of cinnamon oil on varroa behavior. Our experiments were designed to show how varroas' behavior alter in different media. The illustration below respresents how cinnamon oil prevents varroas from attaching male larvae since cinnamon oil contains cinnamaldehyde. Repeated experiments were done and controls groups (glycerol) were used to get more reliables results.

This plate is a regular plate which is not affected by a repellent. We observed the movements of varroa mites.

This plate is a regular plate which is not affected by a repellent. We observed the movements of varroa mites.

This plate includes varroa mites and male bee larvae. We theoretically knew that male bee larvae attract varroa mites. By this sample we observed this theory. Because varroa mites moved towards the larvae.
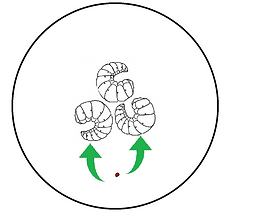

We drop some cinnamaldehyde in the middle of the plate. This plate showed us that varroa mites moved away from cinnamaldehyde.

We applied cinnamaldehyde to surround the edge of the plate. Varroa mites began to gather at the middle of the plate.

We tilled male bee larvae to cinnamaldehyde and put them in the middle of plate. Varroa mites started to move away and began to gather at the edge of the plate. This is the proof of repellent effect of cinnamaldehyde.

We put some glycerol to the middle of the plate. Varroa mites moved through the middle of the plate.

We applied glycerol to surround the edge of the plate. This sample shows us glycerol has no effect on varroa mites and they continued their regular movement.

We put 3 male bee larvae which are tilled to glycerol. The plate showed us that varroa mites continue their moves through the larvae. So, glycerol has no repellent effect on varroa and that why larvae don’t lose their attraction.
Video 1. Short version of behavior experiment
Video 2. Long version of behavior experiment

Table 1. Index of behavior experiment
Materials Used
-
Bee larvae taken from drug-free hives
-
Varroa taken from bee larvae
-
2% concentrated Cinnamaldehyde solution
-
2% concentrated Glycerol solution
-
Glass Plates
-
Brush
Protocols
Transformation Protocol:
1)Throw competent cell on ice
2) Mix ligation product 50 microliter cell+2 microliter plasmid
3)Incubate cells on ice 30 mins
4) Heat shock 55 sec CaCl2, 75 sec RuCl2
Add 900 ml LB
5)Incubate 37 celcius for 80 minute
6)Centrifuge at 3000 po m 10 mın
7)Discard supernatant
8)Resuspend pellet in 100 ml LB
9)Spread cells and wait 14-16 hours in the incubator
LB Broth Protocol:
1)10 gr peptone
2)5 gr yeast extract
3)10 gr NaCl
4)up to 1000ml dH20 & autoclave
LB Agar Protocol:
1)10 gr peptone
2)5 gr yeast extract
3)10 gr NaCl
4)15 gr agar
5)up to 1000ml dH20 & autoclave
6)use 20 gr for 1lt dH2O
ONC Protocol:
1)Put 5 ml LB into a tube.
2)Put a loop of E.coli dH5 aseptic.
3)Seal the tube using parafilm.
4)Place the tube diagonally into a shaker for 14-18 hours.(optimum 14)
Competent Cell Preparation by Calcium Chloride Protocol:
1)Inoculate 1ml ONC to 100 ml LB in a flask.
2)Incubate the flask for 2-4h at 37 ˚C, check via spectrophotometer at 600nm for 0.300..
3)Divide the solution into two falcon tubes(50 ml).
4)Spin them down at +4 ˚C and 5000g for 5min.
5)Discard supernatant.
6)Resuspend the cells with 10 ml cold CaCl2.
7)Put in ice for 10 minutes.
8)Spin the suspension down at +4 ˚C, 5000g for 5 minutes.
9)Discard supernatant.
10)Add 10 ml cold CaCl2 and resuspend the cells.
11)Put the cells in ice for 30 min.
12)Centrifuge at 5000 rpm for 5 min at +4 ˚C.
13)Discard supernatant.
14)Put 1 ml CaCl2 and dissolve pellet.
15)Put it on ice for 5 min. and keep it at +4 ˚C.
Getting the DNA Parts from the Kit Plate:
1)Add 10 microliter of ddH2O into the wanted well.
2)Wait and get the part by pipetting.
3)Make sure to keep stock from these parts since there is so less of them.
4)In case of having too less sample, you can dilute the stock 10:1 with ddH2O.
Midi Prep Plasmid Isolation Protocol:
A)Bacterial culture, harvest, and lysis.
1)Pellet 25 ml (high copy) or 100ml (low copy) overnight LB culture at 6000xg for 15 min at +4˚C.
2)Homogeneously resuspend the bacterial pellet in 4 ml Buffer P1.
3)Add 4 ml Buffer P2, mix thoroughly by vigorously inverting 4-6 times, and incubate at room temperature for 5 min.
4)Add 4 ml Buffer P3, mix thoroughly by vigorously inverting 4-6 times, and incubate on ice for 15 min.
B)Bacterial lysate clearing
5)Centrifuge at ≥20.000 xg for 30 min at +4 ˚C. Re-centrifuge the supernatant at ≥20.000 xg for 15 min at +4 ˚C.
C)Bind, wash, and elute plasmid DNA on QIAGEN-tip.
6)Equilibrate a QIAGEN-tip 100 by applying 4ml Buffer QBT and allow column to empty by gravity flow.
7)Apply the supernatant(step 5) to the QIAGEN-tip and allow it to enter the resin by gravity flow.
8)Wash the QIAGEN-tip with 2x10 ml Buffer QC. Allow Buffer QC to move through the QIAGEN-tip by gravity flow.
9)Elute DNA with 5 ml Buffer QC into clean 2 ml, 15ml or 50ml vessel.
D)Precipitate, wash, and redissolve plasmid DNA
10)Precipitate DNA by adding 3.5 ml room-temperature isopropanol to the eluted DNA and mix. Centrifuge at ≥ 15.000 xg for 30 min at +4℃. Carefully decant supernatant.
11) Wash DNA pelelt with 2ml room-temperature 70% ethanol and centrifuge at ≥ 15.000 xg for 10 min. Carefully decant supernatant.
12) Air-dry pellet for 5-10 min and redissolve DNA in a suitable volume of buffer.
Plasmid Isolation Protocol:
Resuspend the pelleted cells in 250μL of the Resuspension Solution. Transfer the cell suspension to a microcentrifuge tube. The bacteria should be resuspended completely by vortexing or pipetting up and down until no cell clumps remain.
Add 250μL of the Lysis Solution and mix thoroughly by inverting the tube 4-6 times until the solution becomes viscous and slightly clear.
Add 250μL of the Lysis Solution and mix immediately and thoroughly by inverting the tube 4-6 times.
Centrifuge for 5 min to pellet cell debris and chromosomal DNA.
Transfer the supernatant to the supplied GeneJET spin column by decanting or pipetting. Avoid disturbing or transferring the white precipitate.
Centrifuge for 1 min. Discard the flow-through and place column back into the same collection tube.
Add 500μL of the Wash Solution to the GeneJET spin column. Centrifuge for 30-60 seconds and discard the flow-through. Place the column back into the same collection tube.
Repeat the wash procedure using 500μL of the Wash Solution.
Discard the flow-through and centrifuge for an additional 1 min to remove residual Wash Solution. This step is essential to avoid residual ethanol in plasmid preps.
Transfer the GneJET spin column into a fresh 1.5 ml microcentrifuge tube. Add 50μL of the Elution Buffer to the center of GeneJET spin column membrane to elute the plasmid DNA. Take care not to contact the membrane with the pipette tip. Incubate for 2 min at room temperature and centrifuge for 2 min.
Discard the column and store the purified plasmid DNA at -20 ˚C.
80% Glycerol Preparation:
Add 80ml 99.7% glycerol to 20ml demineralized water
Autoclave